What happens when you add a proton?
When you add a proton to a completely empty atom,you get hydrogen. When a proton is added to a atom, the atom becomes stable.
What does it mean when an atom is stable? What does it mean when an atom is unstable?
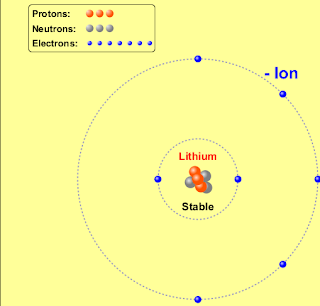
A stable atom is an atom that is able to hold the nucleus together, including the electrons, neutrons, and protons. Unstable atoms are radioactive. They are unable to hold all of their parts together, and therefore radiation is released. Initally, radioactive materials are made of unstable atoms, who do not have enough binding energy to hold themselves together.
How do you make an atom stable? What do you need to do?
In order to make an atom stable, you need to add or subtract a certain amount of protons, depending on the amount of neutrons an atom has. I have noticed that the easiest way to stabilize an atom is to have the same amount of neutrons and protons within it. However, there are some exceptions, like, for example, helium. Helium is an element in the noble gasses family, and it has one neutron and two protons. To my surprise it was still stable!
What is the valence electron pattern as you move across the periodic table?
Valence electrons are the electrons that are placed in the outer shell of an atom. These electrons can become parts of another atom, or can be shared among two atoms. It is when valent electrons "connect" atoms that compunds are created. The valence electron pattern in the periodic table is very simple. When you go across the period, the number of valence electrons increases. When you start a new period, or a new "row", the number of valence electrons decreases, and drops to one. Then, the number starts increasing again.
What happens to the atom when there is more protons, more neutrons, or more electrons?
The atom becomes unstable, but like in the case of helium, there are some exceptions!
What's the difference between a positive and a negative ion?
Ions are formed when an atom, or an element, loses or gains an electron. An ion becomes positively charged, when it loses an electron, because in that case the number of protons is bigger then the number of electrons. An ion is negatively charged if the opposite happens; an atom gains an electron. In that case, there are more electrons then protons.
When you add a proton to a completely empty atom,you get hydrogen. When a proton is added to a atom, the atom becomes stable.
What does it mean when an atom is stable? What does it mean when an atom is unstable?
A stable atom is an atom that is able to hold the nucleus together, including the electrons, neutrons, and protons. Unstable atoms are radioactive. They are unable to hold all of their parts together, and therefore radiation is released. Initally, radioactive materials are made of unstable atoms, who do not have enough binding energy to hold themselves together.
How do you make an atom stable? What do you need to do?
In order to make an atom stable, you need to add or subtract a certain amount of protons, depending on the amount of neutrons an atom has. I have noticed that the easiest way to stabilize an atom is to have the same amount of neutrons and protons within it. However, there are some exceptions, like, for example, helium. Helium is an element in the noble gasses family, and it has one neutron and two protons. To my surprise it was still stable!
What is the valence electron pattern as you move across the periodic table?
Valence electrons are the electrons that are placed in the outer shell of an atom. These electrons can become parts of another atom, or can be shared among two atoms. It is when valent electrons "connect" atoms that compunds are created. The valence electron pattern in the periodic table is very simple. When you go across the period, the number of valence electrons increases. When you start a new period, or a new "row", the number of valence electrons decreases, and drops to one. Then, the number starts increasing again.
What happens to the atom when there is more protons, more neutrons, or more electrons?
The atom becomes unstable, but like in the case of helium, there are some exceptions!
What's the difference between a positive and a negative ion?
Ions are formed when an atom, or an element, loses or gains an electron. An ion becomes positively charged, when it loses an electron, because in that case the number of protons is bigger then the number of electrons. An ion is negatively charged if the opposite happens; an atom gains an electron. In that case, there are more electrons then protons.

Well done Teodora. Your answers were well written and clear. You were able to answer all questions with evidence from using the simulation activity and it was clear that you understood the concepts presented to you.
ReplyDelete